Bare Coulomb potential for hydrogen¶
Using plane waves¶
GPAW has a special PAW setup for hydrogen called ae for
all-electron. It’s not really a PAW setup because it doesn’t make use
of any PAW magic at all — it’s just the bare Coulomb potential
(\(-1/r\)).
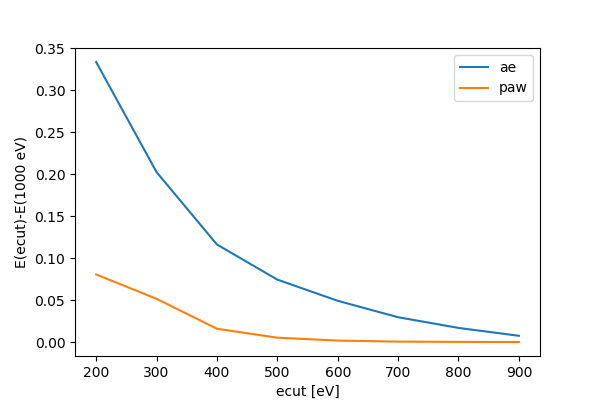
The convergence of the energy as a function of plane-wave cutoff energy will be very slow due to the divergence of the potential at the hydrogen nucleus and also because of the cusp in the wave function:
from ase import Atoms
from gpaw import GPAW, PW
h = Atoms('H', cell=(5, 5, 5))
h.center()
for ecut in range(200, 1001, 100):
h.calc = GPAW(setups='ae',
mode=PW(ecut),
txt=f'H-{ecut}-ae.txt')
e = h.get_potential_energy()
You can look at the energy convergence with this command:
$ ase gui H.ae.txt
Let’s do the same calculation with a PAW setup. Replace the h.calc
= line with this:
h.calc = GPAW(mode='fd', txt='H.paw.txt')
Now the energy is converged much quicker:
Using a 1-d radial grid¶
Since the H atom is spherically symmetric, one can solve the problem on a 1-d grid. You can do that on the command line like this:
$ gpaw atom H -p